edit checks in clinical data management|Enhancing Data Integrity by Applying Edit Checks to Your TMF : Clark Learn how to verify and correct clinical trial data using data validation and edit checks. Explore the techniques, examples, process, and benefits of data validation in CDM. Mini Militia Mod by Sahad Ikr; Mini Militia Mega Mod Apk; . Mini Militia Double Gun Mod Apk; Mini Militia Old Version; Mini Militia Mod Menu Apk; . Mod Apk versions are primarily designed for Android devices. iOS users may need to use other methods. These methods will let them access modified versions of the game.
PH0 · Tips for Writing Effective Edit Checks in eCRFs
PH1 · Practical Guide to Clinical Data Management
PH2 · Ensuring Data Quality: A Comprehensive Guide to Edit Check
PH3 · Ensuring Data Quality: A Comprehensive Guide to Edit Check
PH4 · Enhancing Data Integrity by Applying Edit Checks to Your TMF
PH5 · Edit Checks in Clinical Data Management
PH6 · Edit Checks in Clinical Data Management
PH7 · Edit Checks
PH8 · Edit Check Design Principles
PH9 · Data management in clinical research: An overview
PH10 · Data Validation and Edit Checks
PH11 · Clinical Data Management
PH12 · A cross
Visit Phil Long Ford of Colorado Springs to check out all the models we have on our lot. We have the best deals on Ford cars and trucks. Visit us today! Skip to main content. 1565 Auto Mall Loop Directions Colorado Springs, CO 80920. Sales: 719-413-6467; Service: 719-413-6471; Parts: 719-413-6468;
edit checks in clinical data management*******Learn how to write effective edit checks to ensure data quality and consistency in clinical trials. BioPharma Services offers EDC platforms, data management and biostatistics services for Phase 1 and .Learn how to create and run edit checks on clinical data using a template, a database, or a system. Edit checks are used to validate data quality and consistency throughout the study.Learn how to verify and correct clinical trial data using data validation and edit checks. Explore the techniques, examples, process, and benefits of data validation in CDM. This chapter discusses the use of edit checks in clinical studies, including the purpose of edit checks, types of edit checks, creation processes of edit check .Edit checks are a great mechanism to improve data quality within an electronic data capture (EDC) system. Learn how to determine which edit checks are esse.By applying clinical data management type techniques similar to what we use in EDC, we can run logical checks (going forward, referred to as TMF edit checks), on our eTMF .Learn how to design and implement Edit Check Specifications to ensure data quality and integrity in Electronic Data Capture (EDC) systems. Explore the importance, . The management of clinical data, from its collection during a trial to its extraction for analysis, has become a critical element in the steps to prepare a .Edit check programs are written to identify the discrepancies in the entered data, which are embedded in the database, to ensure data validity. These programs are written .edit checks in clinical data management Enhancing Data Integrity by Applying Edit Checks to Your TMF Our solution enables timely and integrated access to all study data regardless of source, facilitates the review of validation and discrepancy checks and the .The following three levels of data checks should be performed: general clinical data checks, CDISC standard domain checks, and protocol compliance checks. (Doles, 2004) A. Key Variables - All unique key variables in each data set are required. 1. Demographics (DM): Subject identification number is non-missing and unique.
The Society for Clinical Data Management, in their guidelines for good clinical data management practices, states: “Regulations and guidelines do not address minimum acceptable data quality levels for clinical trial data. In fact, there is limited published research investigating the distribution or characteristics of clinical trial data .Clinical Data Management: eCRF & Edit Checks. Background. When I was a Clinical Data Analyst (CDA), my task include coming up with all kinds of data cleaning process to ensure that clinical data entered into the database is clean. One of the strategy is to have Edit Checks in place on the eCRF (electronic case report form) in order to eliminate .Enhancing Data Integrity by Applying Edit Checks to Your TMF Data validation: edit checks, source data verification, and data anonymization Experts in charge: data manager, database designer, quality control associate Clinical data validation is a series of quality tests to ensure accuracy, consistency, legibility, and integrity of information. This includes the following steps. . Carefully designed edit checks can greatly increase efficiency and data quality by automating many data review processes within the clinical database or clinical data management system (CDMS). CDM personnel and members of the study team should collaborate to determine what edit checks should be in place to fulfill study . While EDC edit checks can work well for simple data entry issues, more advanced checks are still needed to ensure overall data quality. . We have presented an integrated solution for clinical data review that helps data managers identify missing, erroneous and inconsistent data, manage queries and organize their workload in a .The Good Clinical Data Management Practices (GCDMP ©) standard provides a reference to clinical data managers in their implementation of high quality Clinical Data Management processes and is used as a guidance tool for clinical data managers when preparing for CDM training and education. We are currently revising the chapters of the . Overview of edit check documentation
In clinical trial, one of the most important tasks facing clinical data management personnel is to produce the electronic Edit Checks specifications for a study. Developing the electronic edit check specification -- and processing the queries that result from them -- is arguably the most vital and time-consuming data cleaning activity data . The data manager may close a query on data that violates an edit check. In these cases, she is overriding the demands of the validation logic, but only after careful consideration and consultation .
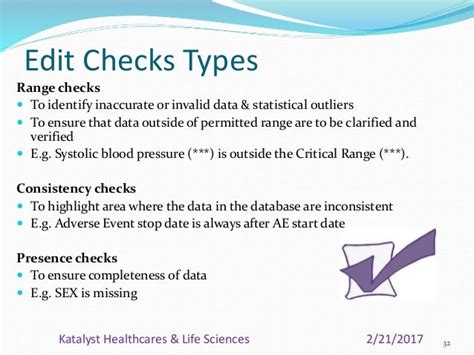
That’s why the ability to perform real-time edit checks, rather than having to wait until a designated moment to view and address queries, can make or break a study’s chances of success, elevating one of the clinical trials challenges. Indeed, this ability can be hard to come by. Safeguards of data quality in an EDC typically involve an . The Society for Clinical Data management, in their guidelines for good clinical data management practices, states: "Regulations and guidelines do not address minimum acceptable data quality levels for clinical trial data. . -based CRFs allow advanced Edit Checks during Data Collection stage to prevent major data cleaning .Edit checks in Clinical Data Management ensure all data meets the previously defined rules. They help to validate the reliability and quality of the data collected. Edit checks are vital to the clinical trial process as they can detect inconsistencies in the data for the most accurate data collection and management.Clinical Data Management (CDM) processes, including Edit Checks could be applied to eTMF management and oversight Employing Edit Checks would potentially result in increased TMF compliance, greater quality compliance, and would be more efficient than our current, manual oversight processes Application of CDM processes could transform . Edit checks can an great mechanism to improve information property within an electronic data capture (EDC) system.Here are many benefits to creating them in electronic case report mailing (eCRFs) so as real-time feedback for site associates as few enter data, soon resolution of data discrepancies, and automated review, allowing . Edit checks may be simple or complex; evaluate a single item or a group of related items; prevent the user from moving on or simply raise a flag. You can learn all about these differences below. The goals of edit checks are universal: higher data quality right from the start! 1. Check yourself. Setting edit checks appropriately is all about .

A clinical trial management system (CTMS) is a type of project management software specific to clinical research and clinical data management. It allows for centralized planning, reporting, and tracking of all aspects of clinical trials, with the end goal of ensuring that the trials are efficient, compliant, and successful, whether .Improving Edit Check Programming: a New Metric. Edit check programming and testing is time consuming and labor intensive. As such, it is efficient to minimize the time spent on programming by getting it right the first time. Historically, data management have measured edit check success as the percentage of edit checks that pass User .
PornHub ist die weltweit führende kostenlose Porno-site. Wähle aus Millionen von Harcore Pornovideos, die schnell und in Höchstqualität streamen, sowie VR Pornos. Die umfangreichste Erwachsenen-site des Internets wird immer besser. Wir haben mehr Pornostars und echte Amateure als jede andere site. Schnell, kostenlos, genau das .
edit checks in clinical data management|Enhancing Data Integrity by Applying Edit Checks to Your TMF